How Much Does It Cost To Build Clinical Trial Management Software

Clinical Trial Management Software Development: A Game-Changer
In the dynamic landscape of healthcare, precision and efficiency are paramount, especially in the realm of clinical trials. Clinical trials play a pivotal role in advancing medical research and innovation, serving as the cornerstone for evaluating the safety and efficacy of new treatments and therapies. However, orchestrating these complex endeavors requires meticulous planning, precise execution, and comprehensive management. This is where Clinical Trial Management Software (CTMS) steps in as a vital tool in the modern healthcare landscape. CTMS empowers researchers, clinicians, and sponsors with the capabilities to streamline trial processes, optimize resource allocation, and ensure regulatory compliance. By digitizing and automating key tasks such as participant recruitment, data collection, and reporting, CTMS revolutionizes the way clinical trials are conducted, accelerating the pace of discovery and improving patient outcomes. In this dynamic and ever-evolving field, CTMS serves as an indispensable ally, facilitating collaboration, enhancing efficiency, and driving transformative advancements in healthcare.
Clinical Trial Management Software serves as the backbone of modern clinical research, streamlining processes, and enhancing collaboration among stakeholders. It empowers researchers, sponsors, and regulatory authorities to efficiently oversee every aspect of a clinical trial, from planning and recruitment to data analysis and reporting.
Benefits of Clinical Trial Management Software
Enhanced Efficiency:
Automation of manual tasks accelerates processes, reducing administrative burden and minimizing errors.
Centralized Data Management:
CTMS provides a unified platform for storing, organizing, and accessing trial-related data, ensuring data integrity and security.
Improved Compliance:
Built-in regulatory compliance features facilitate adherence to industry regulations and standards, reducing the risk of non-compliance.
Real-Time Insights:
Robust reporting and analytics capabilities offer stakeholders actionable insights, enabling informed decision-making throughout the trial lifecycle.

Clinical Trail Management can provide actionable real-time insights
Automated patient recruitment:
CTMS can automate patient recruitment by screening electronic health records and displaying results on a clear-cut dashboard, reducing recruitment time and costs.
Cost reduction with machine learning:
Predictive modeling can estimate the time needed to enroll participants from certain locations, helping manage the process efficiently and keeping costs stable.
Siteless trials:
CTMS allows for siteless trials, where participants can connect, upload, consult, and review data from any location, reducing travel costs and inconvenience for participants.
Reduced oversights and human errors:
CTMS provides performance scoring and visualizations for all studies, reducing the likelihood of oversights and human errors.
Improved collaboration:
CTMS enables better collaboration among team members, allowing them to work together on a single task or study, and access the latest information and track progress.
Real-time data accessibility:
EDC systems within clinical trials provide real-time data accessibility, ensuring accurate and reliable information for effective decision-making.
Automation:
Modern CTMS automates processes, such as data input and collection, to focus on other pressing areas and ensure data integrity.
Key Features of Clinical Trial Management Software
Clinical trial management software (CTMS) offers a wide range of key features to streamline and enhance the management of clinical trials. Some of the essential features include:
Protocol and Clinical Study Documentation Tracking and Management:
This feature allows for the review, management, and authoring of essential study documents, tracking their status throughout their lifecycle, and managing the electronic Trial Master File (eTMF).
Clinical Trial Project Management:
Enables oversight and management of clinical trials based on project activities, timelines, and resources, facilitating communication within the team, managing submissions, and coordinating activities among different departments.
Development and Tracking of Study Forms, Approvals, and Milestones:
Involves managing case report forms (CRF), electronic data capture (EDC) components, key milestones like last patient visit (LPV), regulatory approvals tracking, and more.
Management of Study Financials:
This feature allows for budget projection and tracking, managing grants, processing payments related to study activities, and handling financial disclosures.
Site, Subject, and Team Management:
Provides functionalities for site monitoring, subject enrollment, site visits management, defining roles and responsibilities of all participants in a trial.
Study Management and Clinical Monitoring:
Tracks key information such as CRF collection, clinical research associate (CRA) monitoring frequency, adherence to protocol regimen, supports study documentation workflows, and monitors quality criteria for regulatory compliance.
Investigator Management:
Enables sponsors to manage relationships with investigators, track study data status with CROs and investigator sites, and monitor approvals by Institutional Review Boards (IRB) and Independent Ethics Committees (IEC).
Issue Management:
Allows for tracking, managing, and resolving issues according to good clinical practice (GCP), ensuring ethical standards are maintained throughout the trial in your participant management software.
Clinical Supply Management—Storage and Shipment:
Oversees clinical supply logistics including inventory locations, lots, shipment details, tracks multiple service providers involved in the trial supply chain.
Clinical Trial Data Management: Involves archiving data, warehousing data for analysis, resolving queries related to discrepancies in clinical data ensuring data security throughout the trial process.
Technology Trends in Clinical Trial Management Software
Clinical trial management software (CTMS) is evolving with the integration of advanced technologies to streamline and enhance clinical research. Some of the recent technology trends in CTMS include:
Cloud-based Solutions: Many CTMS providers are offering cloud-based solutions, making it easier for researchers and sponsors to access and manage clinical trial data from anywhere, at any time.
Artificial Intelligence (AI) and Machine Learning (ML): AI and ML are being integrated into CTMS to automate processes, improve data analysis, and enhance decision-making.
Blockchain Technology: Blockchain technology is being used to ensure data security and integrity in clinical trials, as well as to facilitate data sharing and collaboration among stakeholders.
Telemedicine: CTMS is incorporating telemedicine capabilities to enable remote patient monitoring and data collection, improving patient access to care and reducing the need for in-person visits.
EHR Integration: CTMS is integrating with electronic health records (EHRs) to facilitate patient recruitment, data collection, and analysis, improving the overall efficiency of clinical trials.
Regulatory Compliance Tools: CTMS is developing tools to ensure compliance with regulatory standards, such as HIPAA and GDPR, by implementing robust data security measures.
Mobile Applications: CTMS is developing mobile applications to enable real-time data entry, patient engagement, and remote monitoring, improving the patient experience and trial efficiency.
Patient-centric Solutions: CTMS is focusing on developing patient-centric solutions to improve patient engagement, retention, and satisfaction, ultimately leading to better trial outcomes.
Data Analytics and Visualization: CTMS is incorporating advanced data analytics and visualization tools to provide real-time insights and facilitate decision-making during clinical trials.
Customization and Scalability: CTMS is offering customizable and scalable solutions to meet the specific needs of different trials and accommodate growth over time.
Development Process of Clinical Trial Management Software
The development process of Clinical Trial Management Software (CTMS) involves several key stages and considerations to ensure the success of clinical trials. Here is an overview of the development process based on the provided search results:
Understanding User Requirements:
The development process begins with a thorough understanding of user requirements by engaging with stakeholders such as researchers, clinical coordinators, regulatory professionals, and data managers to identify their needs, pain points, and desired features for the software.
Design and Development:
The software development process includes designing user-friendly interfaces, comprehensive training materials, and responsive customer support to ensure that users can effectively utilize the software to support their research.
Testing and Quality Assurance:
Rigorous testing is essential to ensure the functionality, usability, and security of the CTMS. Testing should cover aspects like data accuracy, regulatory compliance, user experience, and system performance.
Deployment:
Once the software is developed and tested, it is deployed in a production environment. Comprehensive training is provided to users on its functionalities, workflows, and best practices to ensure smooth adoption.
Ongoing Support and Maintenance:
Continuous technical support is offered to address user queries, troubleshoot issues, provide updates or enhancements, and incorporate user feedback for continuous improvement of the system.
Continuous Improvement:
Monitoring user feedback and performance metrics helps identify areas for improvement and optimization. Regular updates and enhancements are implemented to address user needs, incorporate new features, and stay compliant with evolving regulatory requirements.
How Much Does It Cost to Build Clinical Trial Management Software
The cost of building clinical trial management software can vary widely depending on factors such as features, complexity, technology stack, development team rates, and project timeline. However, here’s a breakdown of potential costs:
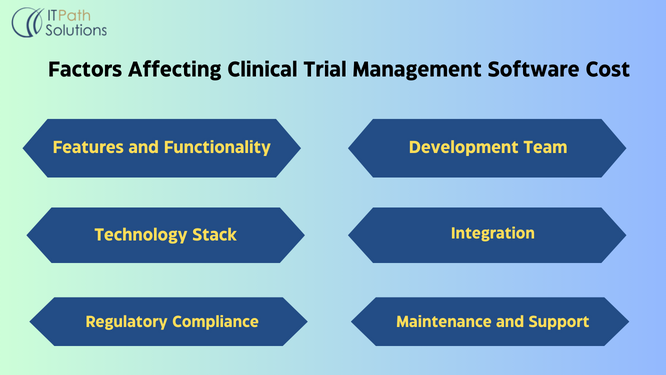
Features and Functionality
The more features and complexity you add, the higher the development costs. Basic functionalities may include participant management, data capture, reporting, and compliance tracking.
Development Team
This includes developers, designers, project managers, and QA testers. Rates vary based on location and expertise.
Technology Stack
Choosing the right technology stack (programming languages, frameworks, databases, etc.) can impact development time and costs. Open-source technologies may reduce licensing fees but require more customization.
Integration
If your software needs to integrate with other systems or databases, additional costs may arise for API development and third-party integration.
Regulatory Compliance
Ensuring compliance with regulatory standards such as HIPAA and GDPR may require additional resources for auditing, documentation, and security measures.
Maintenance and Support
Factor in ongoing maintenance, updates, and technical support post-launch. This may include bug fixes, feature enhancements, and addressing regulatory changes.
IT Path Solutions: Your Trusted Partner For Clinical Trial Management Software Development
At IT Path Solutions, we pride ourselves on being your trusted partner for clinical trial management software development. With years of expertise in healthcare technology, we understand the unique challenges and requirements of the clinical research industry. Our dedicated team of developers, designers, and healthcare experts collaborate closely with you to create tailored solutions that streamline trial processes, enhance data accuracy, and improve overall efficiency.
From participant recruitment and tracking to data management and regulatory compliance, we leverage the latest technologies and best practices to deliver robust, user-friendly software that meets your specific needs. With a focus on security, scalability, and interoperability, we ensure that your clinical trial management software not only meets regulatory standards but also seamlessly integrates with existing systems and workflows. Partner with IT Path Solutions to unlock the full potential of your clinical trials and accelerate the pace of medical innovation.
 Healthcare
Healthcare  Education
Education  Real Estate
Real Estate  Logistic
Logistic  Themes
Themes
 Plugins
Plugins
 Patterns
Patterns